
There are at least 5 basic diy shampoo facts I wish I knew before trying to make a shampoo. I’ve experimented with making shampoos since the days I used to use Castile soap. Most of us have tried everything to make a great shampoo just short of getting a degree in Chemistry! LOL! There may be some things I’ve missed, but here are the Top 5 basic things I wish I knew before attempting to make shampoo.
1- Shampoos are Just Meant to Clean
This seems self-evident, but you have to understand it in the context of trying to dupe commercial products. Shampoos are meant to clean dirt, oils, and debris out of the hair. That’s it.
Shampoos are not meant to condition the hair. They’re not meant to impart shine. They’re not meant to lessen frizz. They’re not meant to “seal” or “repair” split ends. None of the cosmetic puffery I used to believe has helped me make a good shampoo.
Join The Community!
Become a member to access all the formulas and recipes now!
Whenever making anything, start with the goal of the actual product. What is it you want it to do? Stick to that. Trying to do 12 different things with 1 product will cause unnecessary frustration.
Companies who sell commercial products have an enormous amount of resources in comparison to us. They also have sizable marketing budgets, so if they want to convince us they’ve made a 12-in-1 shampoo, they’re going to try. It doesn’t mean we should try to dupe that allegedly amazing product at home. There are simply too many unknowns.
An effective shampoo will remove oils, dirt and debris from the hair. Don’t use more than 1% total oils in shampoos. Vegetable oils are not the reason your hair DOESN’T feel like straw. Nor are they the reason why your hair is shiny after rinse off. The heavy-lifter ingredients we usually run away from at home are performing that job.
I suggest to skip oils altogether in a shampoo unless it’s directly part of how you’ll be marketing the product to your target audience. If your target audience wants a Castor oil shampoo, you gotta use castor oil. Remember, shampoos are meant to clean. Just make sure castor oil is used at less than 1% or else the shampoo could feel ineffective and may not lather well.
From my understanding, the surfactants in the shampoo can’t tell the difference between the buildup on your hair and castor oil anyway. That means it’s going to get washed right down the drain with the dirt and oils.
Bottom line: save all of your expensive additives, extracts and oils for leave-in conditioners, sprays and serums UNLESS you want to do what the big boy’s do: cosmetic puffery (ie “claims”).
2 – Don’t Get Tripped Up By “Claims” Ingredients
As I said before, the recommendation is never to add more than 1% total of oils into a shampoo. Many of the commercial products we used have a long list of great ingredients. What they don’t tell us is the percentage of the ingredient in the actual product.
It’s quite possible many of those great ingredients are added at 0.05% or 0.1% just so the marketing people can make the claim that it’s in the product. “Claims” ingredients are ingredients added at an extremely low percentage in order to market that product to a specific audience. It’s part of that “cosmetic puffery” everybody learned about from Chemist Steve Miller’s (Gabel’s Cosmetics) epic videos about cosmetic manufacturing (1).
Let’s say you know your audience is looking for haircare products with Sugarcane extract because they’ve heard it does something wonderful for the hair. You add 0.1% sugarcane extract to a shampoo. Technically, you can claim it “contains Sugarcane extract”. It’s true…but it’s not the ingredient doing the work of keeping the hair from feeling like straw. And certainly it’s not at a percentage to actually do anything important. Plus, it’ll be washed down the drain because the surfactant ingredients will get rid of it.
Yes, I was taken aback too. But technically they aren’t lying. It’s us, the public, who makes connections which don’t exist.
For example, let’s say you like a “curl defining” shampoo that contains panthenol, and you want to make your own. Some people may make the connection that it’s the panthenol helping with curl definition. Nope. More than likely it’s probably the low pH of the shampoo and possibly the hydrolyzed proteins that are making your coily curls appear more prominent and defined.
So watch out for “claims” ingredients when it comes to trying to dupe a shampoo at home. Stick to what you know makes your hair look and feel good. At home, you’re probably using things like herbal infusions at a high percentage… which is probably why using an infusion of tea, for example, as a shampoo base can make your hair appear shiny temporarily after rinsing.
Bottom line: try to figure out what the “claims” ingredients are and simply ignore them. What I do is find the “parfum” or “fragrance” in the list of ingredients of a commercial product and ignore every extract listed after that. Some of the chemists say to ignore extracts altogether, but let’s move on. 😛
3 – Not All Surfactants Thicken With Salt (Sodium Chloride)
Everybody knows that adding a little salt can thicken some homemade surfactant based shampoos. However, depending on which ingredients you’ve used, salt will not thicken the shampoo and you will have to use a separate thickening ingredient to improve the viscosity.
Many of us have the goal of making sulfate free shampoo because we’ve had bad experiences with bad formulas from major manufacturers. But some of the sulfate free ingredients we use don’t thicken with salt.
Talk to a good chemist to figure out why these ingredients won’t thicken with salt. Here is a little information from UL Prospector about that:
“Salt thickens by reducing micelle charge density, helping to promote the conversion of spherical micelles to rod-shaped micelles. ” (2)
What does that meeeean? For our purposes, it doesn’t matter. What most formulators need to focus on is what to use, how to use it, and what not to use.
Nonionic, sulfate-free surfactants like decyl glucoside and lauryl glucoside don’t thicken well with salt. (3) They may require a separate surfactant-thickening ingredient to get the viscosity we all love to see in a shampoo.
According to IPCS(6), the following classes of surfactants WILL thicken with salt:
*Alkyl sulfates (ex: sodium lauryl sulfate)
*Alkyl ether sulfates (ex: sodium lauryl ether sulfate)
*Fatty acid taurides (ex: sodium cocoyl methyl taurate)
*Fatty acid isethionates (ex: sodium cocoyl isethionate)
The following classes of surfactants WON’T thicken with salt:
*Sulfosuccinate (ex: disodium laureth sulfosuccinate)
*Acyl glutamates (ex: sodium cocoyl glutamate)
*Amphoterics (ex: cocamidopropyl betaine and the hydroxysultaines)
*Nonionic surfactants (ex: alkyl glucosides, decyl glucoside)
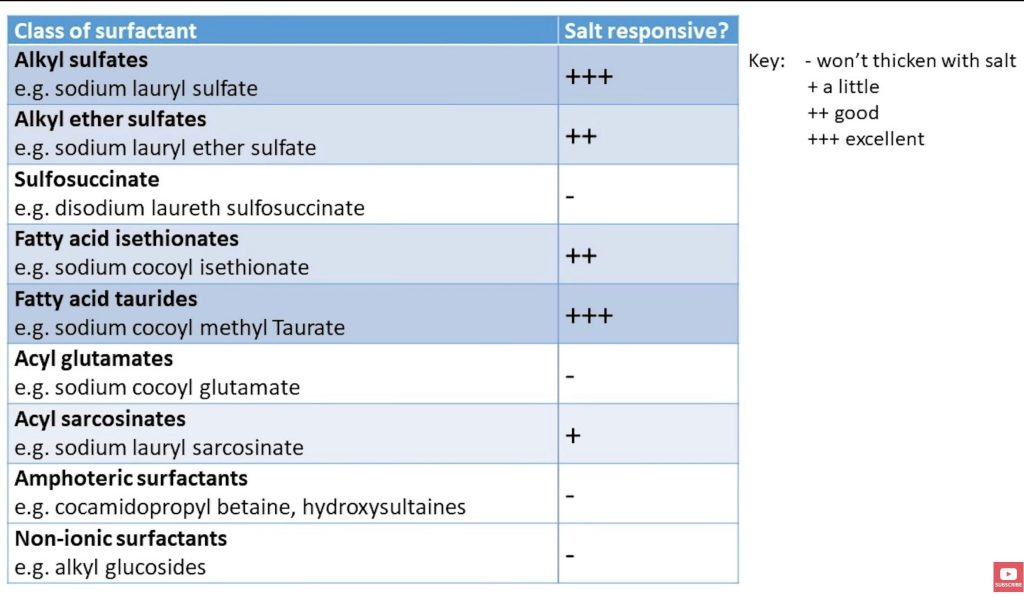
Bottom line: Figure out what ingredients you have (or what you should buy). Then see if you can thicken it with salt, or if you need a separate ingredient to turn it into an attractive shampoo.
Many surfactants we gravitated towards when making homemade shampoo falls under the class of surfactants that won’t thicken with salt. So be ready to keep experimenting with various thickening ingredients.
4 – The Best Shampoos Contain a Combination of Surfactants
One of the most important points of the 5 basic DIY shampoo facts concern the makeup of shampoo. Great shampoos are made up of a combination of surfactant ingredients. Shampoos usually contain an ANIONIC surfactant(s), a NONIONIC surfactant(s) and an AMPHOTERIC surfactant(s). Amphoteric surfactants are also referred to as “zwitterionic” surfactants.
Anionic surfactants have a negative charge. These are your primary surfactants. They are the workhorses that do a lot of the removal of dirt, oil and buildup. However, they can be a little harsh by themselves depending on the percentage. Sodium lauryl Sulfate (SLS) and Ammonium Lauryl Sulfate (ALS) are anionic.
While running away from SLS, a common anionic cleansing surfactant many of us gravitated towards is Sodium C14-16 alpha olefin sulfonate. You’ll know this as “Alpha Olefin” if you’re familiar with some of the formulas at curlytea.com. When I need to make a shampoo that feels like a true, commercial shampoo, I’ll reach for this first.
Another great anionic surfactant is Disodium laureth sulfosuccinate or “Sulfosuccinate”. It’s a “mild” anionic surfactant meant to be used with other surfactants in a shampoo. It said to have good emulsifying and foaming qualities.
I usually mix Alpha Olefin and Sulfosuccinate in one formula. They work well together.
If looking specifically for anionic surfactants, look at the INCI. The anionics often have something like this in their titles:
“sulfosuccinate” (ex: Disodium laureth sulfosuccinate)
“___sulfonate” (ex: Sodium C14-16 alpha olefin sulfonate)
“___taurate” (ex: Sodium Methyl Oleoyl Taurate)
“___sarcocinate” (ex: Sodium Lauroyl Sarcosinate)
” isethionate” (ex: Sodium Cocoyl Isethionate)
There are more but you may be more likely to find these.
Nonionic surfactants have no charge. These are known as secondary surfactants. They’re used to help combat any harshness from the anionic surfactants. For example, the nonionic decyl glucoside is said to be mild and is said to be good at producing lather.
You’ll find nonionic surfactants used heavily in those “baby” shampoos or shampoos for “sensitive” skin. If you want to make gentle shampoos, start with a combo of nonionic cleansing surfactants. They may not work quite as well, or as quickly, as the anionics though.
Amphoteric surfactants (zwitterionic) have “two oppositely charged groups” (4), which means they can behave like anionic or cationic surfactants depending on the pH.(5)
These are the betaines (like Cocamidopropyl betaine and Coco betaine), and the sultaines (like Hempseedamidopropyl hydroxysultaine). They are mild and are often used to thicken shampoo formulas. I’ve specifically used Coco betaine to thicken up a watery shampoo formula. But it also has the added benefit of greatly boosting the lather of the shampoo.
Cocamidopropyl Betaine and Coco Betaine are different. Here are their INCIs.
Cocamidopropyl betaine: Cocamidopropyl betaine, sodium chloride, glycerin, 3-dimethylaminopropylamine, tetrasodium EDTA, methylchloroisothiazolinone, methylisothiazolinone, water
Coco Betaine: Coco betaine, water, sodium chloride, 1,2-benzisothiazolin-3-one
Using a combination of these in your formulas will provide a better cleansing effect. However, you don’t have to mix your own. There are ready made surfactant blends that already contain a mix of anionic, nonionic and amphoteric cleansing surfactants. An example of a premixed blend of surfactants is Iselux Ultra Mild.
Iselux Ultra Mild should be familiar if you’re a subscriber at curlytea.com. I use it all the time because of it’s convenience. Add water, a preservative, your chosen additives, a refatting agent, and any polyquats/-cones. No heating required.
When I want to make Iselux Ultra Mild into a clarifier, I add another anionic surfactant (like Alpha Olefin). When I want more lather, I’ll add Cocamidopropyl Betaine or Coco Betaine.
When I want a squeaky clean feeling, I’ll use Alpha Olefin, any nonionic surfactant and add an amphoteric like Hempseedamidopropyl hydroxysultaine (ColaTeric HBS) to the mix.
Bottom line: using a combination of various surfactant ingredients gives the best result.
5 – Don’t Shy Away from Polyquats (and Cones)
Ok wait. Hear me out! Through experimentation I’ve come to understand that the ingredients doing a lot of the extra work are the ones with a bad reputation. Both polyquaternium and many silicones actually improve shampoo formulas in a positive way.
For example, Polyquaternium-7 is a cationic conditioning agent that adds more slip to a shampoo formula. It makes a shampoo feel better when applying it to the hair (lubricity). It’s great to use when you want the final shampoo to remain clear. Polyquats also have anti-static and film-forming capabilities.
Another great addition is Polyquaternium-10. It’s a cationic conditioning, detangling and thickening agent that works well even with cleansing surfactants. It sticks to hair proteins and helps with frizz. It helps in the deposition of silicone on the hair too.
A small percentage (0.2%) is fine. Many naturals worry about buildup with polyquats. But in a shampoo? At 0.2%?? I don’t think you need to be bothered by that. It’s much more likely that excess use of your post- shampoo products (like leave-in conditioners, treatments, gel stylers, etc), are causing build up. The lack of shampooing the hair as needed also causes build up.
Washing the hair with a sulfate shampoo every once in a while will help remove buildup. Don’t completely run away from it. Use it like a clarifier for best results.
Silicone ingredients actually perform a service for dry damaged hair; especially for my usually ‘high porosity’ curls. I still can’t bring myself to use straight dimethicone in a formula on purpose. Haha! But I’ve started to incorporate Amodimethicone into some of my shampoo and conditioner formulas because my hair is normally high porosity.
If you have high porosity hair, definitely consider incorporating polyquat 7, polyquat 10 and/or amodimethicone. If you’re unsure, start at very low percentages.
Bottom line: Don’t run away from the very ingredients that may be doing the heavy-lifting of keeping the hair from feeling like straw; or keeping the hair from feeling like a tangled, frizzy mess after rinse off.
Please don’t be afraid to make and use real, full bodied shampoo on your curls as necessary. I ditched co-washes a long time ago because I make, use and test so many conditioners and serums. I need to get those products off the hair and prep it for the next product.
I’m still experimenting with shampoos like the rest of you. But I wanted to present some general info I’ve been able to piece together so far from that research and experimentation.
Happy DIYing!
- Private Label Haircare P1
- UL Prospector – Thickening Surfactant Based Products
- Lotioncrafter
- UL Prospector – Surface Active Agents
- BulkChemicals2Go.com
- Institute of Personal Care Science
Video links
+Steve Miller – The Truth About Hair Products: How They Are Made
+Steve Miller – How to create your own brand, product line, private labeling
+Perry Romanowski – Cosmetic Science Webinar – 7 Essentials
+The Institute of Personal Care Science – How to thicken surfactants with salt
 CURLYTEA
CURLYTEA


![Ep 20: 5 Basic DIY Shampoo Facts [PODCAST]](https://www.curlytea.com/wp-content/uploads/ct_thumb_720x405_5BasicThingsAboutShampoo-2-1.jpg)








